| Long-term
Safety of Investigational CCR5 Antagonist Vicriviroc in Treatment-experienced
Patients Schering-Plough's investigational CCR5 antagonist vicriviroc has demonstrated potent activity against HIV in laboratory studies and clinical trials of treatment-experienced patients.
More than 200 patients received vicriviroc in Phase II trials. In a poster presented at the 48th International Conference on Antimicrobial Agents and Chemotherapy (ICAAC 2008), taking place this week in Washington, DC, researchers described long-term safety data from this population. Participants in studies ACTG 5211 and VICTOR-E1 were allowed to continue taking vicriviroc on an open-label basis after completing the initial 48-week randomized dosing period. A total of 205 patients with advanced HIV disease were enrolled in the 2 trials, and 196 received more than 12 weeks of vicriviroc. The drug was started at doses ranging from 5 to 30 mg, but all patients eventually escalated to 30 mg once-daily, as supported by safety and efficacy findings. In the present analysis, data were pooled for all participants, regardless of initial dose, since no safety differences were seen across dose groups. Taken together, most study participants (84%) were men, 73% were white, 40% were Hispanic/Latino, about two-thirds were from North America, and the mean CD4 count was about 200 cells/mm3. AIDS complications, malignancies, infections, and liver-related abnormalities were evaluated. Changes in HIV RNA levels and CD4 counts were based on available data at defined intervals. Results
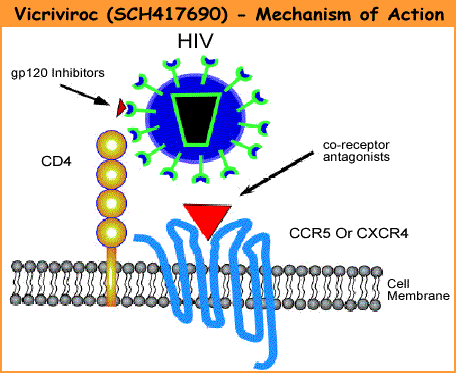
According to the investigators, "These data represent the longest treatment duration and clinical experience reported to date for CCR5 antagonist." "Vicriviroc demonstrated excellent tolerability, few HIV complications, and no clearly vicriviroc-related toxicity," they concluded. "Sustained antiviral effect with prolonged maintenance of improved CD4 counts was observed." In this study, vicriviroc was "not associated with hepatotoxicity, seizures, or ischemic cardiovascular events," they added. "These long-term results demonstrate that vicriviroc added to an optimized background therapy may provide durable viral suppression and sustained elevated CD4 counts in treatment-experienced HIV-infected patients," said study investigator Jihad Slim in a pres release issued by Schering-Plough. "Importantly, vicriviroc was well tolerated, with some patients continuing on treatment for up to four years, and it was not associated with increased liver, [central nervous system] or cardiovascular adverse events or with an increased incidence of malignancy in this patient population." Vicriviroc is currently being studied in 2 large international Phase III trials, VICTOR-E3 and VICTOR-E4, which have completed enrollment with more than 850 treatment-experienced patients. It is also being evaluated as first-line therapy for treatment-naive patients in an ongoing Phase II study. Reanalysis Using Enhanced Trofile Assay In a related study, investigators reanalyzed data from ACTG 5211 using a new, more sensitive version of Monogram Biosciences' Trofile HIV tropism assy. CCR5 antagonists like vicriviroc interfere with one of the 2 coreceptors HIV uses to enter cells. HIV tropism -- or which coreceptor the virus uses -- is determined by a tropism test. Only patients with exclusively CCR5-tropic virus, rather than CXCR4-tropic or dual/mixed strains, are considered eligible to use CCR5 antagonists. However, the original Trofile test may have misclassified some patients with low levels of non-CCR5-tropic virus. Researchers
used the enhanced Trofile assay to determine coreceptor usage in 114 patients
who had CCR5-tropic virus at study screening according to the older test. Using
the enhanced test, 89 patients were found to have CCR5-tropic virus, while 25
had dual/mixed virus. Greater reductions in HIV RNA were observed in vicriviroc
recipients who had CCR5-tropic virus at both study screening and study entry,
compared with those who had CCR5-tropic HIV at screening but dual/mixed virus
at study entry and those who had dual/mixed virus at screening. LM Dunkle, WL Greaves, MC McCarthy, and others. Long term vicriviroc safety. 48th International Conference on Antimicrobial Agents and Chemotherapy (ICAAC 2008). Washington, DC. October 25-28, 2008. Abstract H-1269. Z Su, JD Reeves, A Krambrink, and others. Response to vicriviroc (VCV) in HIV-infected treatment-experienced subjects using an enhanced Trofile HIV co-receptor tropism assay: reanalysis of ACTG 5211 results. 48th International Conference on Antimicrobial Agents and Chemotherapy (ICAAC 2008). Washington, DC. October 25-28, 2008. Abstract H-895. Other
source |
The
material posted on HIV and Hepatitis.com about ICAAC 2008 and IDSA 2008 is not
approved by nor is it a part of ICAAC 2008 or IDSA 2008. |
![]()