Novel
Entry Inhibitor VIR-576 Shows Promise in Early Study
 |
 |
 |
 |
 |
 |
 |
| SUMMARY:
A novel type of HIV entry inhibitor dubbed VIR-576,
which blocks HIV's ability to insert its gp41 fusion
peptide into the outer membrane of a host cell,
demonstrated promising safety and efficacy in a
small clinical study published in the December
20, 2010 issue of Science Translational Medicine.
VIR-576 did not show cross-resistance with other
antiretroviral drugs -- including other entry inhibitors
-- and is unlikely to promote resistance itself,
according to study investigators. |
|
 |
 |
 |
 |
 |
 |
 |
By
Liz Highleyman
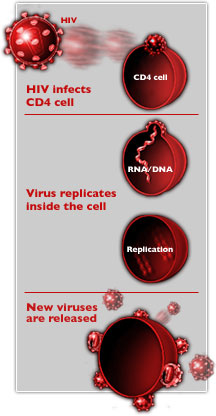 In
order to infect host cells, HIV
must grab onto and fuse with the cell's membrane, allowing
virus components access the cell's interior. A new therapy
based on a natural substance produced by the body may block
this interaction.
In
order to infect host cells, HIV
must grab onto and fuse with the cell's membrane, allowing
virus components access the cell's interior. A new therapy
based on a natural substance produced by the body may block
this interaction.
As
described in a media advisory from the American Association
for the Advancement of Science (publisher of Science Translational
Medicine), Wolf-Georg Forssmann from Hannover Medical
School in Germany and an international team of colleagues
conducted a Phase I/II clinical trial to test whether VIR-576
can suppress HIV replication.
VIR-576
is a variant form of a fragment of alpha-1-antitrypsin designated
as virus-inhibitory peptide, or VIRIP. It binds to HIV's hydrophobic
fusion peptide gp41 -- described as the "sticky"
end of its outer envelope -- preventing the virus from inserting
itself into a host cell's membrane to initiate infection.
The novel compound works differently than the 2 approved entry
inhibitors, enfuvirtide
(Fuzeon or T-20) and maraviroc
(Selzentry).
The
study included 18 treatment-naive participants with HIV viral
loads of at least 10,000 copies/mL and CD4 T-cell counts of
at least 350 cells/mm3. They received injections of one of
3 doses of VIR-576 (0.5, 1.5, or 5.0 grams/day) as monotherapy
for 10 days, before commencing combination antiretroviral
therapy (ART).
Results
 |
At
the end of treatment, VIR-576 reduced mean plasma HIV
RNA by 0.06 log copies/mL in the 0.5 g/day arm, 0.30 log
in the 1.5 g/day group, and 1.23 log in the 5.0 g/day
group. |
 |
At
the highest dose, VIR-576 reduced average viral load by
about 95%. |
 |
Efficacy
was variable among individuals, however, ranging from
0.30 to 1.90 log in the high-dose group. |
 |
There
was a significant correlation between viral load reduction
and plasma concentrations of VIR-576. |
 |
No
changes in CD4 cell counts were seen during this short
treatment period. |
 |
VIR-576
was generally well-tolerated. |
 |
No
major adverse effects were reported. |
 |
The
most frequent adverse events were mild-to-moderate constipation,
headache, and fever, which were not dose-related. |
 |
2
study participants experienced moderate allergic reactions,
which resolved after stopping the drug. |
Based
on these findings, the investigators wrote, "Our results
are proof of concept that fusion peptide inhibitors suppress
viral replication in human patients, and offer prospects for
the development of a new class of drugs that prevent virus
particles from anchoring to and infecting host cells."
VIRIP-related
compounds do not show cross-resistance with other antiretroviral
drugs, including enfuvirtide, they elaborated in their discussion.
Furthermore, because the gp41 fusion peptide is "highly
conserved," or unable to tolerate much genetic mutation,
it is likely to have a high barrier to resistance itself.
Unlike the CCR5 co-receptor targeted by maraviroc, all strains
of HIV-1 rely on gp41 to enter cells.
But the large VIR-576 peptide must be injected, which limits
its convenience and attractiveness to HIV patients with other
treatment options. Investigators are now exploring small-molecule
oral agents that work by a similar mechanism.
"Further clinical development of fusion peptide inhibitors
seems warranted and may provide a useful addition to the current
armamentarium of antiretroviral drugs, particularly for salvage
therapy of individuals infected with multiresistant HIV-1
strains and for the risk reduction of transmission between
an HIV-infected mother and her fetus during birth," the
study authors concluded.
This
type of approach may also be effective against other enveloped
viruses that use fusion peptides, such as influenza virus,
Ebola, and hepatitis B and C.
Investigator
affiliations: Department of Immunology and Rheumatology, Hannover
Medical University, Hannover, Germany; VIRO Pharmaceuticals
GmbH & Co, Hannover, Germany; Mediconomics GmbH, Hannover,
Germany, Department of Chemistry,University of Patras, Rion-Patras,
Greece; CBL-Patras, Patras, Greece; Departamento de Biología
Físico-Química, Centro de Investigaciones Biológicas,
Consejo Superior de Investigaciones Científicas, Madrid,
Spain; Institute of Molecular Virology, University Hospital
of Ulm, Ulm, Germany; Departamento de Química, Facultad
de Farmacia, Universidad San Pablo CEU, Madrid, Spain.
1/7/11
Reference
W-G Forssmann, Y-H The, M Stoll, and others. Short-Term Monotherapy
in HIV-infected Patients with a Virus Entry Inhibitor against
the gp41 Fusion Peptide. Science Translational Medicine
2(63): 63re3 (Abstract).
December 20, 2010.
Other
Source
AAAS. Advance Information for the 22 December 2010 Issue
of Science Translational Medicine. Media advisory.
December 20, 2010.
