HIV
Protein Unveils Vaccine Target
March
30, 2011 -- An international study headed by a UC Davis scientist
describes how a component of a potential HIV vaccine opens like
a flower, undergoing one of the most dramatic protein rearrangements
yet observed in nature. The finding could reveal new targets
for vaccines to prevent HIV infection and AIDS. A paper describing
the work was published online this week in the journal Proceedings
of the National Academy of Sciences.
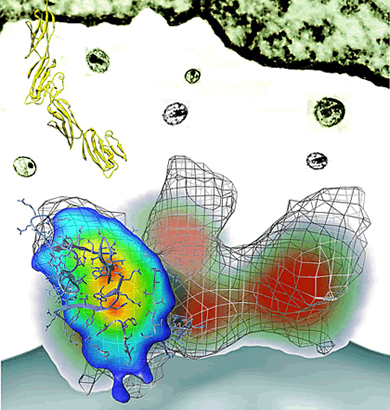 |
This
side view of the HIV envelope protein shows a virus
(bottom) and a T cell (top). The mesh shows its normal
shape, the green/red/blue shading shows its changed
shape.
(R. Holland Cheng/UC Davis graphic). |
|
In
the new study, researchers from the U.S., Sweden and France
explored the structure and behavior of the HIV envelope protein
complex, which could potentially serve as a component of a vaccine
aimed at eliciting the human immune system to generate antibodies
against HIV.
"By opening up these less exposed regions, we might be
able to raise more broadly cross-reactive antibodies to HIV,"
said R. Holland Cheng, professor of molecular and cellular biology
at UC Davis and senior author of the study.
HIV infects a type of white blood cell called the CD4 T-cell,
weakening the immune system and leading to AIDS. HIV attaches
to these cells through the envelope protein complex, which is
made up of three gp120 proteins and three gp41 proteins, Cheng
said.
First, the gp120 protein attaches to a CD4 protein on the victim
cell's membrane. Then it uses gp41 to punch a hole through the
membrane.
UC Davis graduate student Carlos Moscoso and project scientist
Li Xing, working in Cheng's laboratory, used a cryoelectron
microscope to study the structure of the complex and how it
changes when it is exposed to a piece of the CD4 protein. A
cryoelectron microscope derives three-dimensional images of
complex protein structures from samples frozen in liquid nitrogen.
They found that when the HIV protein complex attaches to a CD4
protein, it rotates and flattens, exposing more of the gp41
proteins in the middle -- probably allowing the gp41 protein
to get closer to the cell membrane so it can lock on.
It also potentially exposes an area of the virus that would
be vulnerable to attack by the immune system, Cheng said. If
a person were vaccinated and had antibodies to such a protein
region, they might be able to stop the virus at the point of
invading the CD4 T cell.
The gp120 protein itself varies considerably between strains,
so it has been difficult to make an effective vaccine against
it. But these hidden protein regions vary less between different
strains of HIV, Cheng said.
Cheng's group is part of the HIV Research and Design consortium
formed by the National Institutes of Health to pursue new targets
for HIV vaccines. In future work, the consortium plans to test
potent antibodies from HIV-positive people who have survived
without developing AIDS to see if the antibodies recognize the
new potential vaccine targets.
Investigator affiliations: Department of Molecular and Cellular
Biology, University of California, Davis, CA; Novartis Vaccines
and Diagnostics Inc., Cambridge, MA; Karolinska Institutet,
Structural Virology, Clinical Microbiology/University Hospital,
Stockholm, Sweden; Commissariat à l'énergie atomique
et aux énergies alternatives, Institut de Biologie et
Technologies de Saclay, Service d'Ingénierie Moléculaire
des Protéines, Gif-sur-Yvette, France.
The envelope protein complex was prepared by Novartis Diagnostics
and Vaccines Inc. of Cambridge, Mass. The work was funded by
the NIH.
